Milliequivalent Calculation in Pharmacy
The milliequivalent is the amount, in milligrams, of a solute equal to 1/1000 of its gram-equivalent weight.
Milliequivalent (mEq), is commonly used by clinicians, physicians, pharmacists, and manufacturers to express the concentration of electrolytes in solution.
Conversion of concentrations in the form of milliequivalent to concentrations in percentage strength, milligrams per milliliter (mg/mL) or any other terms, begins with calculation of the number of milliequivalents of drug.
Milliequivalent Calculation Process
1. Determine the molecular weight (mol. wt.)
2. Calculate the equivalent weight (Eq. wt.)
3. Determine the milliequivalent weight, which is 1/1000 of the equivalent weight.
4. Convert to desire concentration
Calculation Formula
To calculate the equivalent weight:
- Equivalent weight = Molecular weight ÷ Valence
To convert milligrams (mg) to milliequivalents (mEq):
- mEq = (mg x Valence) ÷ molecular weight
To convert milliequivalents (mEq) to milligrams (mg):
- mg = (mEq x molecular weight) ÷ Valence
To convert milliequivalents per milliliter (mEq/mL) to
milligrams per milliliter (mg/mL):
- mg/mL= (mEq/mL x molecular weight) ÷ Valence
Calculation Example
1. What is the concentration, in percent w/v, of a solution containing 2 mEq of potassium chloride per milliliter?
Molecular weight of KCl = 39 (K+) + 35.5 (Cl−) = 74.5
Equivalent weight (Eq. wt.) of KCl = mol. wt. ÷ valence = (74.5 ÷1) = 74.5 g
Milliequivalent weight = 74.5 g ÷ 1000 = 0.745 g or 74.5 mg
Drug quantity = 0.0745 g/mEq × 2 mEq = 0.149 g of drug
Concentration = (0.149 g drug ÷ 1 mL) = (x g drug ÷ 100 mL)
x = (14.9 g/100 mL) × 100% = 14.9%
2. A physician prescribes 10 mEq of potassium chloride for a patient. How many milligrams of KCl would provide the prescribed quantity?
Molecular weight of KCl = 39 (K+) + 35.5 (Cl−) = 74.5
Valence = 1
mg = (mEq x mol. wt.) / Valence
mg = (10 x 74.5) / 1 = 745 mg
3. If a patient is prescribed 300 mg of potassium chloride, what is the corresponding mEq?
mEq = mg x Valence / mol. wt.
mEq = (300 x 1) / 74.5 = 4.03
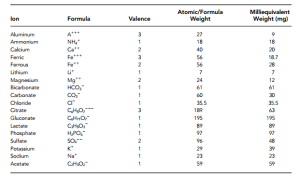
Valences, Atomic Weight/Molecular Weight and Milliequivalent Weights of Few Common Ions

Read also:





